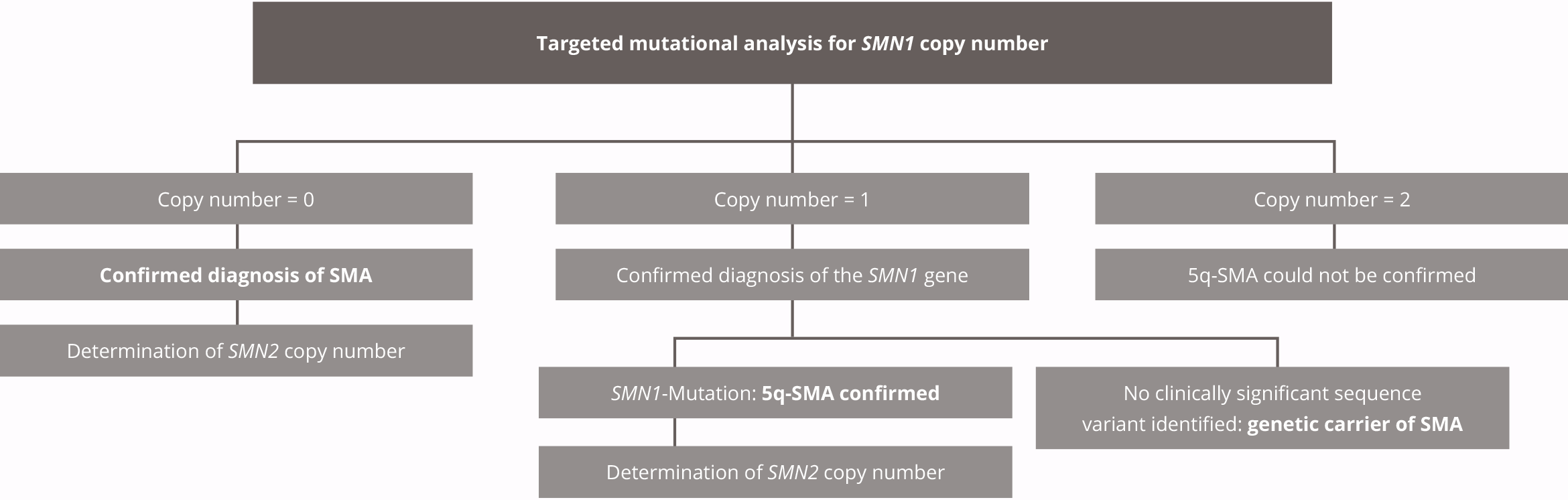
Our service is ideal for identification of SMA in at-risk patients showing specific clinical symptoms or for an individual or family member who has a family history of SMA.
We provide a SMA Sampling Kit which includes:
- Dried Blood Spot card (DBS)
- Informed Consent Form (ICF)
- Envelope for the return of the Sampling Kit (including ICF and DBS card) to ARCHIMEDlife Laboratories
- Instructions for Blood Sampling and Shipment
After sample collection is complete, please return the Sampling Kit (including the DBS card and ICF) to ARCHIMEDlife Medical Laboratories using the shipping instructions provided. Diagnostic results will be available within a few working days.